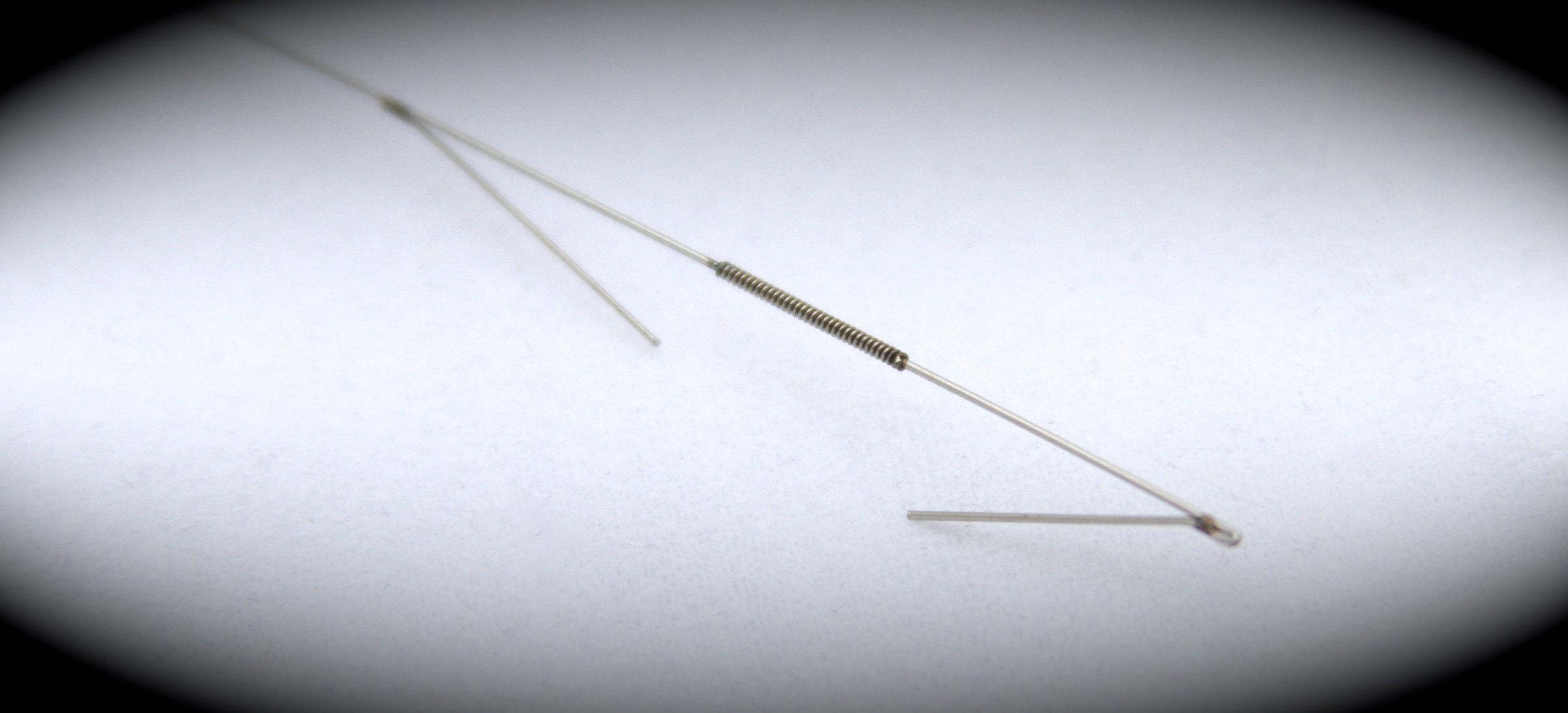
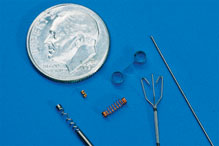
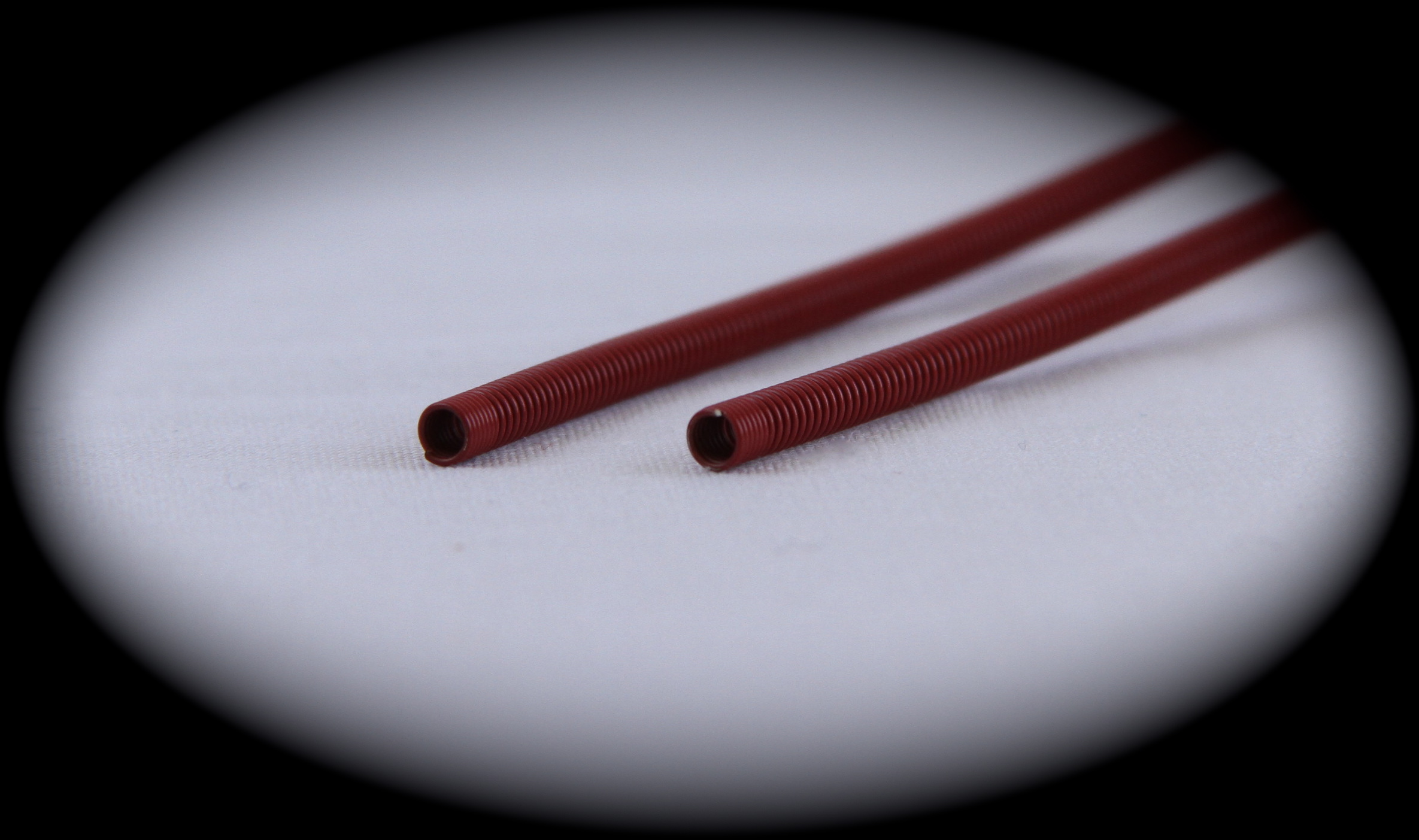
In 2014, Exacto has hired dozens of production personnel to keep up with our growing programs in the medical side of our business. In addition, our custom built equipment for these special programs is mostly made in-house to meet our stringent customer requirements. The machines we use need to be custom built as no job is the same and this type of equipment is not available to purchase. Bending wire to a certain shape does not come easy in medical, especially when you’re adding components to the product and holding the toughest requirements that the Food and Drug Administration (FDA) requires.
The FDA is revising the current good manufacturing practice (CGMP) requirements for medical devices and incorporating them into a quality system regulation. The quality system regulation includes requirements related to the methods used in, and the facilities and controls used for, designing, manufacturing, packaging, labeling, storing, installing, and servicing of medical devices intended for human use. This action is necessary to add preproduction design controls and to achieve consistency with quality system requirements worldwide. This regulation sets forth a framework for device manufacturers to follow and gives them greater flexibility in achieving quality requirements.
The FDA requires detailed studies, protocols, and documentation. Customers also require performance qualifications and operational qualifications for projects, requires a lot of studies, protocols, and documentation of processes, all of which is done by Exacto. Checking dimensions of the devices, checking the worst case scenarios and stacked tolerances require a lot more work, written and documented procedures on equipment. These required documents and studies are produced to the FDA along with the projects. Exacto has all the measuring equipment, using which precise measurements can be taken (can be measured to the microns). All these help Exacto to meet and exceed the requirements set by the FDA. We are also investing in new quality and associated equipment to check the products so that they meet the new and changing required specifications.
Whenever a sponsor intends to conduct a study that is not covered by a protocol already contained in the IND, the sponsor can submit to the FDA a protocol amendment containing the protocol for the study. Such study begins if the following conditions are met:
- The sponsor has submitted the protocol to FDA for review
- The protocol has been approved by the Institutional Review Board (IRB) with responsibility for review and approval of the study in accordance with the requirements of part 56. The sponsor may comply with these two conditions in either order.
Learn more about our capabilities on our website.